- Overview
- Universal iPS
- iPS Differentiation
- Functional Gene Design and Screening
- Bioreactor-based Cell Processing
- Drug Development for UCMSC
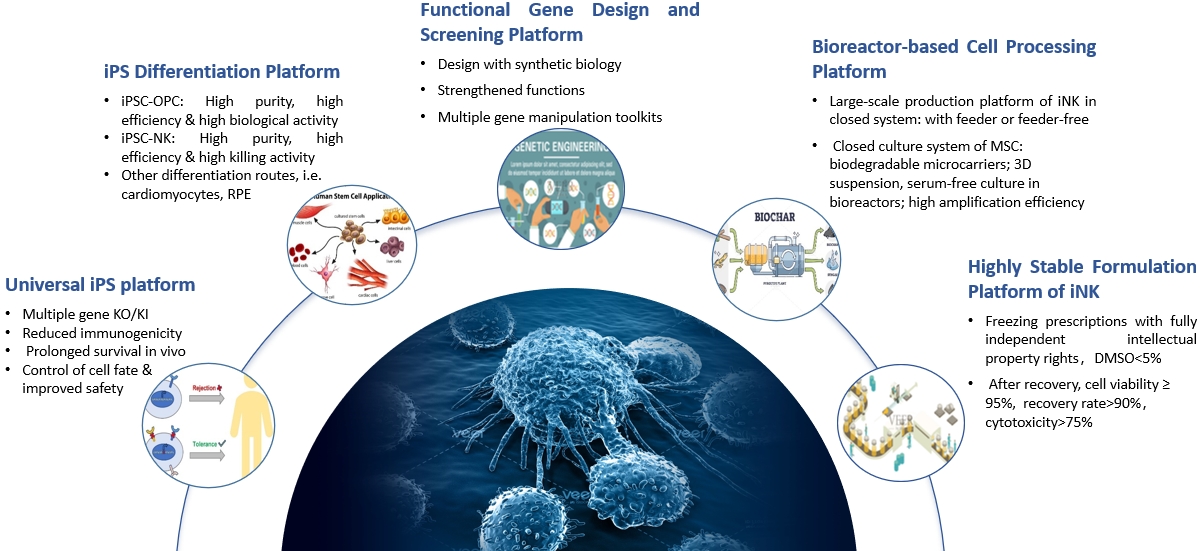
Universal iPS platform
- Multiple gene KO/KI
- Reduced immunogenicity
- Prolonged survival in vivo
- Control of cell fate & improved safety
Our universal iPS cell lines utilizes multiple innovative strategies, including knock-out and knock-in of relevant genes, to reduce immunogenicity when administered into the patient. This ensures much less immune attack from the patient’s immune system and enables prolonged cell survival and functionality after transplantation. Besides, our universal iPS cell lines incorporates a switch that can be used to clear iPS-derived cells whenever the patient is exposed to a potential risk, thus enabling higher safety with the therapy.
iPS Differentiation Platform
- iPSC-OPC: High purity, high efficiency & high biological activity
- iPSC-NK: High purity, high efficiency & high killing activity
- Other differentiation routes, i.e. cardiomyocytes, RPE
We have developed proprietary approaches that can precisely modulate the differentiation process from iPS cells. Our target cell portfolio includes neural progenitor cells, cardiomyocytes, natural killer cells and some others. Each of them is promising in related medical indications, such as neural disorders, cancer and autoimmune diseases, especially when they are combined with our universal iPS platform and additional functional genes. Particularly, we have developed optimized industry-scale process for many of the differentiation and expansion routes, which can be quickly adapted to pre-clinical and clinical development.
Functional Gene Design and Screening Platform
- Design with synthetic biology
- Strengthened functions
- Multiple gene manipulation toolkits
Our differentiated cells may not be used for therapeutic purpose alone. Instead, we will take advantage of our in-depth understanding of science to design and screen functional genes that can improve the functionality of target cells. For instance, a chimeric antigen receptor (CAR) or a chimeric switch receptor can be engineered onto iPS-derived natural killer cells for efficient tumor cell killing. Other state-of-the-art scientific findings also enable us to ‘arm’ our MSCs or iPS-derived cells. To this end, we have a complete set of toolkit with abundant gene delivery or editing tools, including but not confined to lentivirus/retrovirus-mediated delivery, CRISPR/Cas9 or Cas12-mediated gene editing, piggyBac transposon system, etc. These tools will be selected based on the features and requirements of the pipeline.
Bioreactor-based Cell Processing Platform
- Large-scale production platform of iNK in closed system: with feeder or feeder-free
- Closed culture system of MSC: biodegradable microcarriers; 3D suspension, serum-free culture in bioreactors; high amplification efficiency
We have been dedicated to upgrading our cell culture into large-scale process, with or without the use of microcarriers. We use multiple types of bioreactors, including spinner flasks, STBR system and WAVE bag system. For example, we have achieved 1000 fold expansion of our iPS-derived natural killer cells in bioreactors, while sustaining the phenotypes and functions of the cells.
Drug Development for UCMSC
- CMC
- Pre-clinical studies
- IND for a broad range of indications
- Clinical development
We have established a whole process for the development of MSC-based therapeutic products throughout CMC, pre-clinical studies, IND application and conducting clinical trials. Based upon this platform, we have successfully obtained four IND approvals from CDE, including liver cirrhosis, ankylosing spondylitis, acute respiratory distress syndrome (ARDS) and burns. IND application for some other indications are still ongoing. Phase I clinical trials for some of the indications have been nearly completed, with some pipelines demonstrating promising clinical benefit. Overseas filing is also undergoing.
