aCGT’s ‘Umbilical Cord Mesenchymal Stem Cell Injection for the Treatment of Chronic Obstructive Pulmonary Disease’ has obtained IND approval from National Medical Products Administration (NMPA)
On February 7, 2024, during the Chinese New Year celebration, aCGT officially obtained the clinical trial IND approval from the Center for Drug Evaluation (CDE) of the National Medical Products Administration (NMPA), with its Class 1 new drug ‘Umbilical Cord Mesenchymal Stem Cell Injection’ (Acceptance No.: CXSL2300795). This marks the fifth stem cell clinical trial project approved by CDE for aCGT.
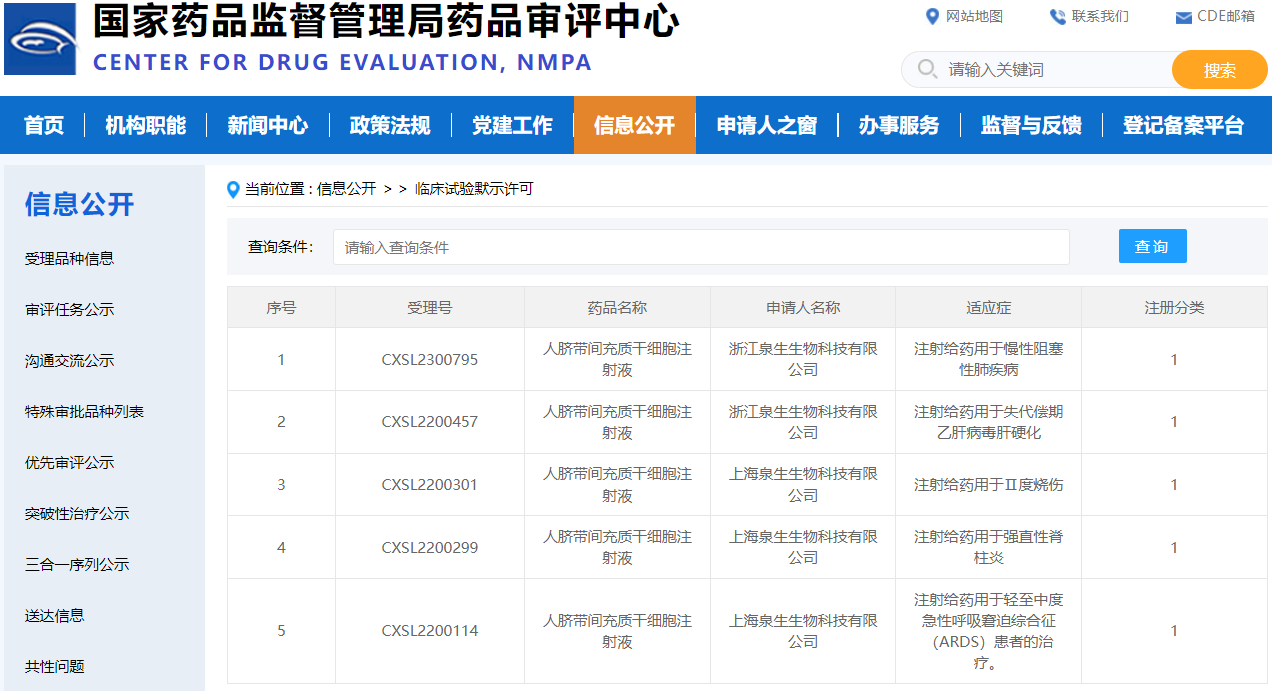
The fifth clinical trial IND approval not only signifies the recognition of aCGT's research and technological capabilities by NMPA but also solidifies aCGT's leading position in the field of stem cell therapeutics development. In the harsh investment climate of the biopharmaceutical industry in 2023, aCGT has been rapidly advancing at a surprising pace, while still facing more difficulties and challenges. In the future, we hope that aCGT will not only achieve more remarkable achievements in the field of stem cell therapy but also continue to establish new standards and new heights in areas such as iPSC and immune cells.
Chronic Obstructive Pulmonary Disease (COPD)
01 Chronic Obstructive Pulmonary Disease (COPD)
Chronic obstructive pulmonary disease (COPD) is a heterogeneous lung condition characterized by chronic respiratory symptoms (such as dyspnea, cough, and sputum production) and persistent (often progressive) airflow limitation due to airway abnormalities (chronic bronchitis, bronchiolitis) and/or alveolar abnormalities (emphysema). The diagnostic criteria for COPD include persistent airflow limitation even after bronchodilator inhalation, indicated by a forced expiratory volume in one second (FEV1) to forced vital capacity (FVC) ratio <0.7 or below the lower limit of normal predicted values, and the exclusion of other diseases that can cause airflow limitation.
COPD is the most common chronic respiratory disease in China, with a high prevalence and heavy disease burden. In 2018, the results of the "China Pulmonary Health Study in Adults" led by Academician Wang Chen showed that the prevalence of COPD in adults aged 20 and above in China was 8.6%, and it reached 13.7% in people aged 40 and above. It is estimated that nearly 100 million patients have COPD in China, indicating a continued high incidence of COPD in the country. The World Health Organization (WHO) predicts that the prevalence of COPD will continue to rise over the next 40 years, with over 5.4 million deaths from COPD and related diseases annually by 2060.
02 The risk factors and pathogenesis of COPD are as follows:
The diverse risk factors contributing to COPD can be summarized as a combination of individual susceptibility and environmental influences:
1. Individual factors include genetic predisposition, age, gender, lung growth and development, bronchial asthma, and airway hyperresponsiveness. COPD exhibits genetic susceptibility, with severe deficiency of α1-antitrypsin being associated with the development of emphysema in nonsmokers. Age is a risk factor for COPD, with higher prevalence rates observed in older individuals.
2. Environmental factors encompass tobacco smoke, biomass smoke, air pollution, occupational dust, infections, and chronic bronchitis. Smoking is the most significant environmental risk factor for COPD. Respiratory infections play a crucial role in the onset and exacerbation of COPD, with viral and/or bacterial infections commonly triggering acute exacerbations.
The pathogenesis of COPD is complex and not fully elucidated. Inhaling harmful particles or gases such as tobacco smoke can induce airway oxidative stress, inflammatory responses, and imbalance of protease/antiprotease pathways, among other mechanisms contributing to the development of COPD. Various inflammatory cells participate in airway inflammation in COPD, including macrophages, neutrophils, as well as Tc1, Th1, Th17, and ILC3 lymphocytes. Additionally, autoimmune regulatory mechanisms, genetic risk factors, and factors related to lung development may also play significant roles in the occurrence and progression of COPD.
03 The main treatment modalities for COPD and their shortcomings are as follows:
The treatment of COPD mainly includes three aspects:
1. Reducing exposure to irritants such as smoking, inhaling toxic gases, or dust.
2. Pharmacological therapy: Bronchodilators are the cornerstone of first-line treatment for COPD, often administered via inhalation. Combination therapy with inhaled corticosteroids can be considered on the basis of using one or two long-acting bronchodilators.
3. Non-pharmacological therapy: Non-pharmacological interventions, in conjunction with pharmacological treatment, are essential components of stable COPD management. These include pulmonary rehabilitation, home oxygen therapy, and surgical interventions (lung transplantation, surgical lung volume reduction, etc.).
Although current treatments can alleviate COPD symptoms and improve patients' quality of life, they cannot halt the progression of COPD or repair damaged lung tissue and function. Additionally, they struggle to reverse the deterioration of lung function. Therefore, there is an urgent need to develop novel and effective treatment strategies for COPD.
04 Stem Cell Therapy for COPD
Multiple preclinical studies have confirmed the beneficial therapeutic effects of stem cells (mainly focused on bone marrow mesenchymal stem cells, adipose-derived mesenchymal stem cells, and umbilical cord mesenchymal stem cells) in animal models of COPD. Several clinical trials investigating the safety and efficacy of mesenchymal stem cell therapy for COPD have provided a solid research foundation for subsequent stem cell therapies.
The main mechanisms of action of stem cell therapy for COPD are as follows:
1. Inhibition of inflammatory reactions: Chronic obstructive pulmonary disease (COPD) is a chronic inflammatory disease involving multiple cells and factors. Mesenchymal stem cells can decrease levels of inflammatory factors, reducing the occurrence of inflammation, possibly through paracrine mechanisms.
2. Correction of protease-antiprotease imbalance: Mesenchymal stem cells may correct the imbalance of protease-antiprotease by activating the positive feedback loop of inflammation and inhibiting protease release.
3. Inhibition of cell apoptosis: Mesenchymal stem cells can inhibit cell apoptosis through various pathways.
4. Suppression of oxidative stress: Oxidative stress plays a significant role in the development of COPD. Mesenchymal stem cells can reduce oxidative stress levels and exert a protective effect on cells. They can also differentiate into alveolar epithelial cells through activation of the Wnt signaling pathway.
Future Prospects of Stem Cell Therapy
aCGT's independently developed Class 1 new biological drug, ‘Umbilical Cord Mesenchymal Stem Cell Injection’, has completed preclinical pharmacology and toxicology studies in accordance with the requirements for Class 1 new biological drugs for therapeutic use. From the perspectives of clinical demand, quality control, non-clinical effectiveness and safety, as well as past clinical safety, all support its entry into clinical trials. According to the clinical trial data conducted by aCGT, stem cells have demonstrated good safety and shown certain unique and superior efficacy in related diseases.
aCGT will always prioritize patient needs, persist in launching new drug development, and further improve related research in strict accordance with the requirements of the National Medical Products Administration, GMP, and GCP. High-quality advancement of clinical research on stem cell products will be ensured, continuously enhancing product safety, stability, and efficacy. This effort aims to actively contribute to safeguarding the health and well-being of the public and improving quality of life.