The NMPA approved aCGT's the forth project "Human Umbilical Cord Mesenchymal Stem Cell Injection for the Treatment of Decompensated Hepatitis B Virus Cirrhosis" to enter clinical trials
On December 14, 2022, Asia Cell Therapeutics (Zhejiang) Co., Ltd. (aCGT), independently developed a new biological class 1 drug "Human Umbilical Cord Mesenchymal Stem Cell Injection" (NO. CXSL2200457). It has officially obtained the implicit approval of drug clinical trials from the NMPA, which is also aCGT's the fourth stem cell clinical trial project. Source: Official website of NMPA The IND obtained the implicit approval for clinical trials, marking another major victory and breakthrough on the road of independent innovation in the research and development of new stem cell drugs, which is of great significance to the entire field of stem cell therapy. In the future, aCGT will continue to strive to explore and move forward and move to new heights on the road of cell drug therapy. The decompensated stage of liver cirrhosis refers to the advanced stage of liver disease caused by various chronic liver damage, characterized by portal hypertension and severe liver damage, and patients often die due to multiple organ failure caused by ascites, primary peritonitis, gastrointestinal bleeding, hepatic encephalopathy, hepatorenal syndrome and sepsis. According to the Global Burden of Disease report, there were 1.32 million deaths due to cirrhosis worldwide in 2017, accounting for 2.4% of global all-cause deaths. The largest cause of cirrhosis is hepatitis B virus (HBV) infection, which is endemic worldwide, but the intensity of HBV infection varies greatly in different regions. According to the WHO, there are about 257 million people with chronic HBV infection worldwide, with 68% in Africa and the Western Pacific. In the Asia-Pacific region, HBV (51.3%) was the leading cause of cirrhosis, followed by ALD (20.8%) and hepatitis C virus (HCV) (15.7%), and metabolism-related fatty liver disease (NAFLD) and other causes (12.1%). At present, there are about 70 million people with chronic HBV infection and about 10 million people with chronic HCV infection in China. According to this estimate, there are about 7 million (or 0.5%) patients with liver cirrhosis in China. For the treatment of patients with liver cirrhosis, the existing treatment methods are mainly drugs for the cause, anti-liver fibrosis drugs, albumin supplementation, diuresis, endoscopic sclerosis or banding, blood purification (artificial liver) and vascular intervention. Although these treatments can slow the progression of the disease, they cannot completely reverse the decline of liver function in all patients. At present, liver transplantation remains the most effective treatment for decompensated cirrhosis. However, due to the lack of donor liver sources, only a few patients are eligible for transplantation. In 2018, a total of 6,272 liver transplants were completed in China, which is far from meeting the clinical needs of the treatment of decompensated liver cirrhosis. The main cause of liver cirrhosis in China is viral hepatitis, and there is no effective treatment to reverse liver cirrhosis, and the decompensated stage of liver cirrhosis refers to the terminal stage of this type of liver disease. Persistent HBV replication is a risk factor for the progression of cirrhosis, and it is extremely difficult to effectively control the progression. In addition, there are more complications in the decompensated stage of liver cirrhosis, which makes it more difficult to treat patients during this period, and the prognosis is also poor, so the treatment of decompensated stage of hepatitis B viral cirrhosis has always been a difficult problem in clinical treatment, and better treatment methods need to be developed urgently. In the past, it was believed that mesenchymal stem cells can be differentiated into hepato-like cells with normal hepatocyte function after implantation into the damaged liver tissue of patients with decompensated liver cirrhosis through the hepatic artery or portal vein, which can replenish the damaged liver tissue and improve liver function. With the further understanding of the mechanism of stem cell therapy for liver disease, it is currently believed that stem cells change the tissue microenvironment through paracrine mechanisms more important than the transdifferentiation of hepatocyte-like cells. For example: MSCs can exert local effects through paracrine function, provide nutrients and an environment conducive to proliferation and repair for the liver, promote the proliferation of damaged liver and liver vascular regeneration, inhibit the proliferation and migration of immune cells to the liver, and regulate the liver and systemic immune inflammatory response, thereby reducing liver damage. MSCs can induce the production of regulatory T cells (Tregs) by secreting IL-10, transforming growth factor β (TGF-β), prostaglandin E2, etc., inhibit excessive inflammatory response, reduce liver inflammation, improve liver microenvironment, inhibit hepatocyte apoptosis, and promote hepatocyte regeneration, inhibit the activity of hepatic stellate cells by secreting IL-10 and TNF-α, or promote the apoptosis of stellate cells through the Fas/FasL pathway, thereby inhibiting the formation of liver fibrosis. So far, stem cell transplantation has been used to treat patients with cirrhosis of various causes, and most studies have shown that stem cells can improve liver biochemistry. For the clinical research of stem cells in the decompensated stage of liver cirrhosis, most of them are for cirrhosis caused by HBV, HCV and ALD, and the clinical evidence is relatively sufficient. The bio-class 1 new drug "Human Umbilical Cord Mesenchymal Stem Cell Injection" independently developed by aCGT has completed preclinical pharmacy, pharmacology and toxicology studies in accordance with the requirements of Class 1 new drugs for therapeutic biological products, and has supported its entry into clinical trials in terms of clinical needs, quality controllability, non-clinical efficacy and safety, and previous clinical safety. The fourth CDE clinical trial implies that it is not only the country's affirmation of aCGT, but also means that aCGT will bear greater social responsibilities and obligations. ACGT will always start from the needs of patients, insist on launching new drug research and development, further improve relevant research in strict accordance with the relevant requirements of the GMP & GCP, promote clinical research of stem cell products with high quality, ensure and continuously improve the safety, stability and effectiveness of products, and make positive contributions to protecting the life, health and well-being of the masses and improving the quality of life.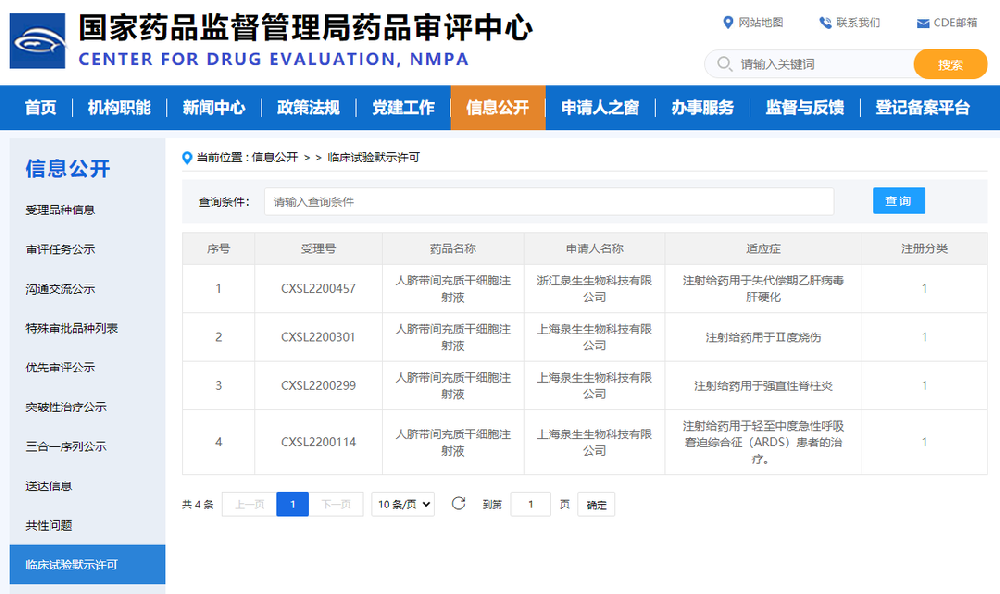
About decompensated hepatitis B virus cirrhosis
01 Current status of decompensated hepatitis B virus cirrhosis
02Traditional treatment of decompensated hepatitis B virus cirrhosis
03Mechanism of stem cell therapy for decompensated hepatitis B virus cirrhosis
Future outlook for stem cell therapy