NMPA officially approved aCGT's second "Human Umbilical Cord Mesenchymal Stem Cell Injection" to enter clinical trials
Great news! On September 9, 2022, on the occasion of the Mid-Autumn Festival, good news spread frequently, Asia Cell Therapeutics (Zhejiang) Co., Ltd.. (aCGT) independently developed a class I biological product "Human Umbilical Cord Mesenchymal Stem Cell Injection" The application for Investigational New Drug (IND) was approved by the CDE (NO.CXSL2200299). Source: Official website of NMPA This is the second indication project of aCGT after the approval of the first indication (acute respiratory distress syndrome,ARDS) of "Human Umbilical Cord Mesenchymal Stem Cell Injection" in May 2022, which is mainly used for the treatment of ankylosing spondylitis (AS), which is also the first stem cell treatment project for AS officially approved by the CDE in China. This marks a new milestone in the independent innovation of new stem cell drug research and development. At the same time, the rapid expansion of indications is testing aCGT, and aCGT will continue to strive to move forward and move towards a higher new dimension. PATHOGENY PART 1 Ankylosing spondylitis (AS) is an inflammatory disease of unknown cause, with a very complex pathogenesis, mainly invading the sacroiliac joints, spinal bony processes, paraspinal soft tissues and peripheral joints, and can be accompanied by extra-articular manifestations, and in severe cases, spinal deformity and ankylosis can occur. According to statistics, there have been more than 5 million cases of AS in China, and about one-20 have caused severe disability, and the probability of male disease is much higher than that of women. The specific pathogenesis of AS is still unclear, but it is mostly thought to be related to immune dysfunction and tissue damage. At present, the treatment of AS is relatively simple, which is mainly divided into drug treatment and surgical treatment. The main goals of treatment are to relieve the symptoms and signs, restore physical function, prevent joint damage, improve the patient's quality of life, and prevent complications of spinal diseases. Clinically, nonsteroidal anti-inflammatory drugs (NSAIDs) and tumor necrosis α factor α (TNF-α) inhibitors are the mainstays of treatment for ankylosing spondylitis, along with sulfasalazine, glucocorticoids, and patient education (eg, physical activity, proper lifestyle and sleep position). In recent years, interleukin receptor blockers and some drugs that inhibit new bone formation have received increasing attention and become the focus of research, including the IL-6 receptor inhibitor sarilumab, the IL-17 receptor inhibitor Cosentyx, the IL-12/23 receptor inhibitor ustekinumab, and the Wnt signaling pathway inhibitor. In addition, there are surgical methods such as spine surgery, artificial total hip replacement, etc. However, none of the above treatment measures can delay the radiological progression of AS and restore the structural damage of patients, and it is difficult to achieve the goal of curing the disease. Drug treatment strategies for AS patients (Babaie, F. et al. Immunol. Lett.2018) TREATMENTPART 2 With the continuous development of stem cell theory and technology, new cell therapies have begun to be applied in clinical practice. Accumulating evidence suggests that mesenchymal stem cell transplantation is an ideal cell therapy option for human autoimmune diseases. Umbilical cord mesenchymal stem cells are derived from the umbilical cord (waste product after birth), have no ethical issues, low immunogenicity, and have multidirectional differentiation potential, and can play immunomodulatory functions by upregulating the function of Treg cells to inhibit T cell proliferation, inhibit B cell proliferation and differentiation, regulate NK cell activity, and prevent dendritic cells (DCs) maturation. Specifically, the mechanism of action of mesenchymal stem cells (MSCS) is mainly divided into the following points: 01 MSCs have the functions of differentiation, regeneration and repair Mesenchymal stem cells are involved in the regeneration of damaged tissues and cells after they are injected into the patient through intravenous infusion, and mesenchymal stem cells have the characteristics of aggregation to the diseased synovium, thereby promoting the repair of damaged joint tissues. 02 MSCs have immunomodulatory functions Mesenchymal stem cells can exert unique immunomodulatory functions, and can effectively inhibit the proliferation and activation of T lymphocytes by adding autologous and allogeneic mesenchymal stem cells to the peripheral blood lymphocyte culture system. 03MSCs have chemotaxis Mesenchymal stem cells can effectively inhibit the release of inflammatory mediators by exerting chemotaxis, thereby reducing inflammation and improving clinical symptoms in patients. EXPECTATIONPART 03 Based on the regeneration, repair, immune regulation and paracrine characteristics of stem cells, Quansheng Biotech's "Human Umbilical Cord Mesenchymal Stem Cell Injection" can be used for the treatment of a variety of major and difficult diseases, including ARDS, AS and other indications. The Class I biological product "Human Umbilical Cord Mesenchymal Stem Cell Injection", independently developed by aCGT, has completed preclinical pharmacy, pharmacology and toxicology studies in accordance with the requirements of Class 1 new drugs for therapeutic biological products, and can support it to enter clinical trials in terms of clinical needs, quality controllability, non-clinical efficacy and safety, and previous clinical safety. The two consecutive IND clinical approvals mark another major progress in the research and development of new drugs for aCGTs' "Human Umbilical Cord Mesenchymal Stem Cell Injection".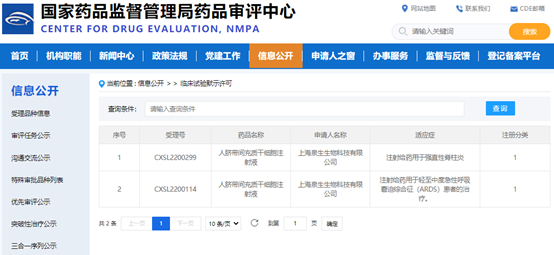
01 Causes of ankylosing spondylitis
02Traditional treatment for ankylosing spondylitis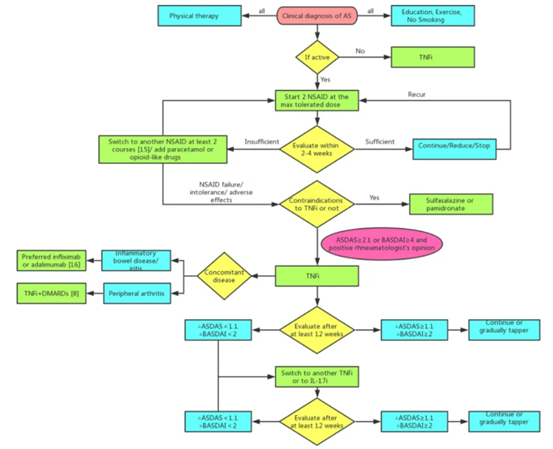


With the gradual opening of the scope of indications, aCGT will rely on its rich R&D experience, start from the needs of patients, insist on launching new drug research and development, accelerate the application of new drugs for more refractory diseases, and further promote the use of "human umbilical cord mesenchymal stem cell injection" for the treatment of more diseases, with the ultimate goal of benefiting the majority of patients and solving more "unmet clinical needs".